Medical geneticists and molecular pathologists play a major role in the growing fields of genomic testing and molecular diagnostics. Reimbursement for professional interpretation of these tests has fallen through the cracks of a complex system.
Native Video Player

FMV for Interpretation of Molecular Diagnostic & Genomic Tests
YouTube Video Player
Medicare reimburses laboratory tests using two different fee schedules. These include the Medicare Physician Fee Schedule (MPFS) and the Clinical Laboratory Fee Schedule (CLFS).
The MPFS focuses on services that are performed and billed by physicians. The MPFS includes many anatomical lab tests that are interpreted by anatomical pathologists. Laboratory service codes on the MPFS often include separate global, professional (26), and technical (TC) reimbursement rates. The bifurcation of reimbursement rates for professional and technical services allows for separation between professional physician services and distinct non-professional laboratory services.
In contrast, the CLFS focuses on services that are often performed using automated instruments that may not require the interpretation of a physician. CLFS services can be billed by laboratories for which there are no physicians nor medical directors involved in operations.
In the 2013 Final Rule of the MPFS, Medicare decided to include new molecular pathology codes on the CLFS instead of the PFS.[1] At that time, Medicare determined that most molecular pathology codes did not require the services of a physician. While this frees up molecular pathology services to clinical laboratories that are not operated by pathologists, it also limits the billing and compensation options when medical geneticist physician services are needed to interpret and annotate molecular pathology test results.
The service code used for physician interpretation of molecular pathology tests (G0452) was reimbursed at about $51 by Medicare during 2025. This amount has proven to be inadequate reimbursement. The American Medical Association’s RVS Update Committee administered surveys to 58 pathologists. These surveys identified 27 minutes of intra-service time for this service. A full-time medical geneticist who is 100% productive during a 2,000-hour work year would find it difficult to achieve market compensation levels at Medicare reimbursement rates (4,000 interpretations x $51 = $204,000 reimbursement).
As a result, some clinical laboratories have arranged to compensate medical geneticists under direct contracts for these interpretation and annotation services. Under these arrangements, the physicians agree to only be compensated by the laboratory and waive their ability to bill insurance separately.
These arrangements create some potential for illegal kickbacks, because medical geneticists regularly refer patients to laboratories for genomic tests and molecular diagnostic tests.
This raises the question: What is Fair Market Value for medical geneticist interpretation and annotation services?
National Reimbursement (UHC PPO)
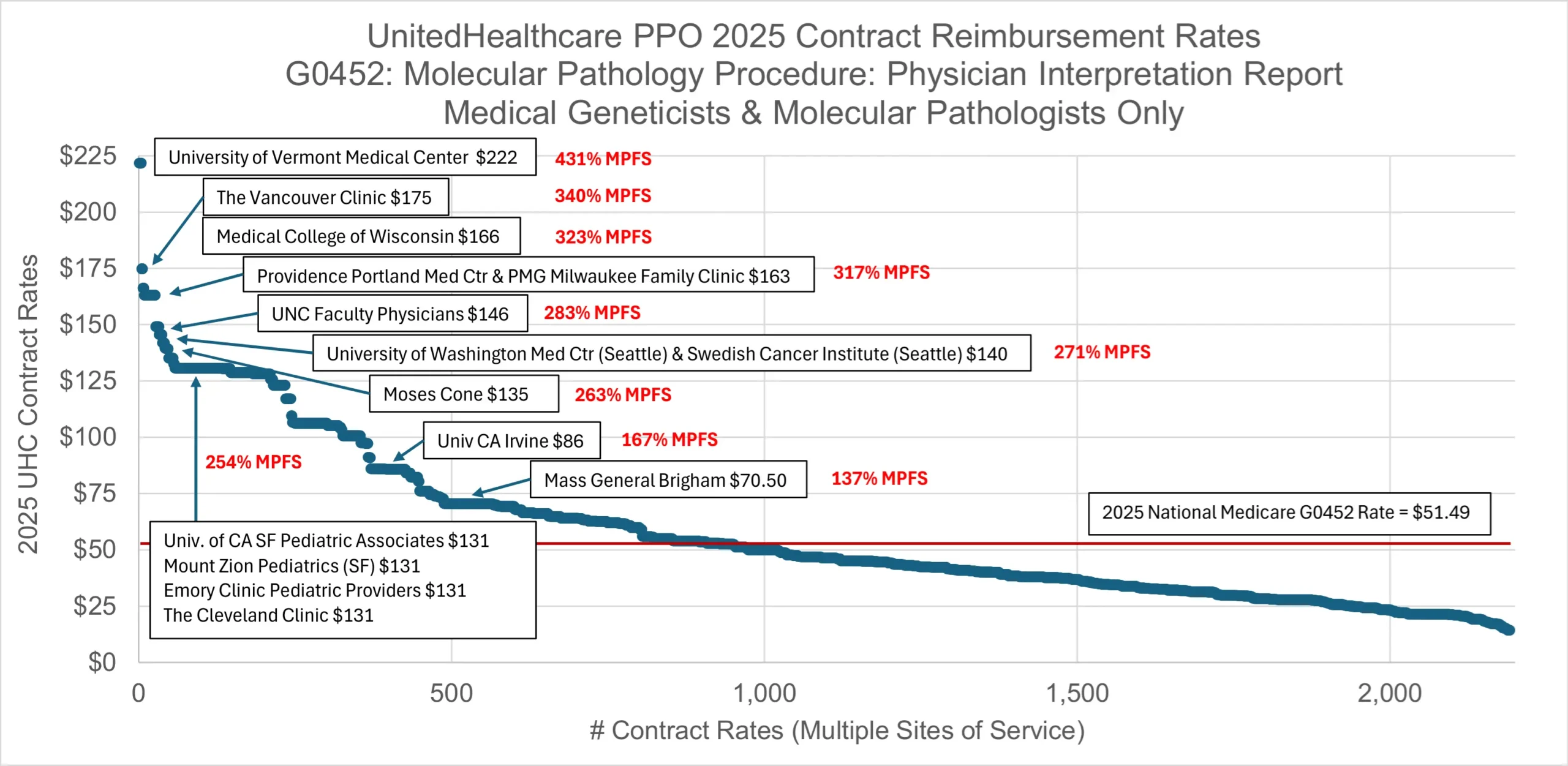
Analysis of reimbursement rates for G0452 for medical geneticists and molecular pathologists has revealed a wide range of commercial rates. Review of reimbursement rates in UnitedHealthcare’s national PPO health plan reveals that United’s median national rate for G0452 is less than Medicare’s national rate ($46.41 vs $51.49).
Despite this, many large employers of medical geneticists and molecular pathologists have negotiated much higher reimbursement rates for G0452 in UnitedHealthcare’s PPO health plan. Mass General Brigham and the University of California-Irvine have negotiated 137% and 167% of the MPFS, respectively. The Cleveland Clinic and Emory Clinic both negotiated about 254% of the MPFS. Many different university medical centers have negotiated between 271% and 323% of the MPFS.
Local Reimbursement: Blue Cross of California
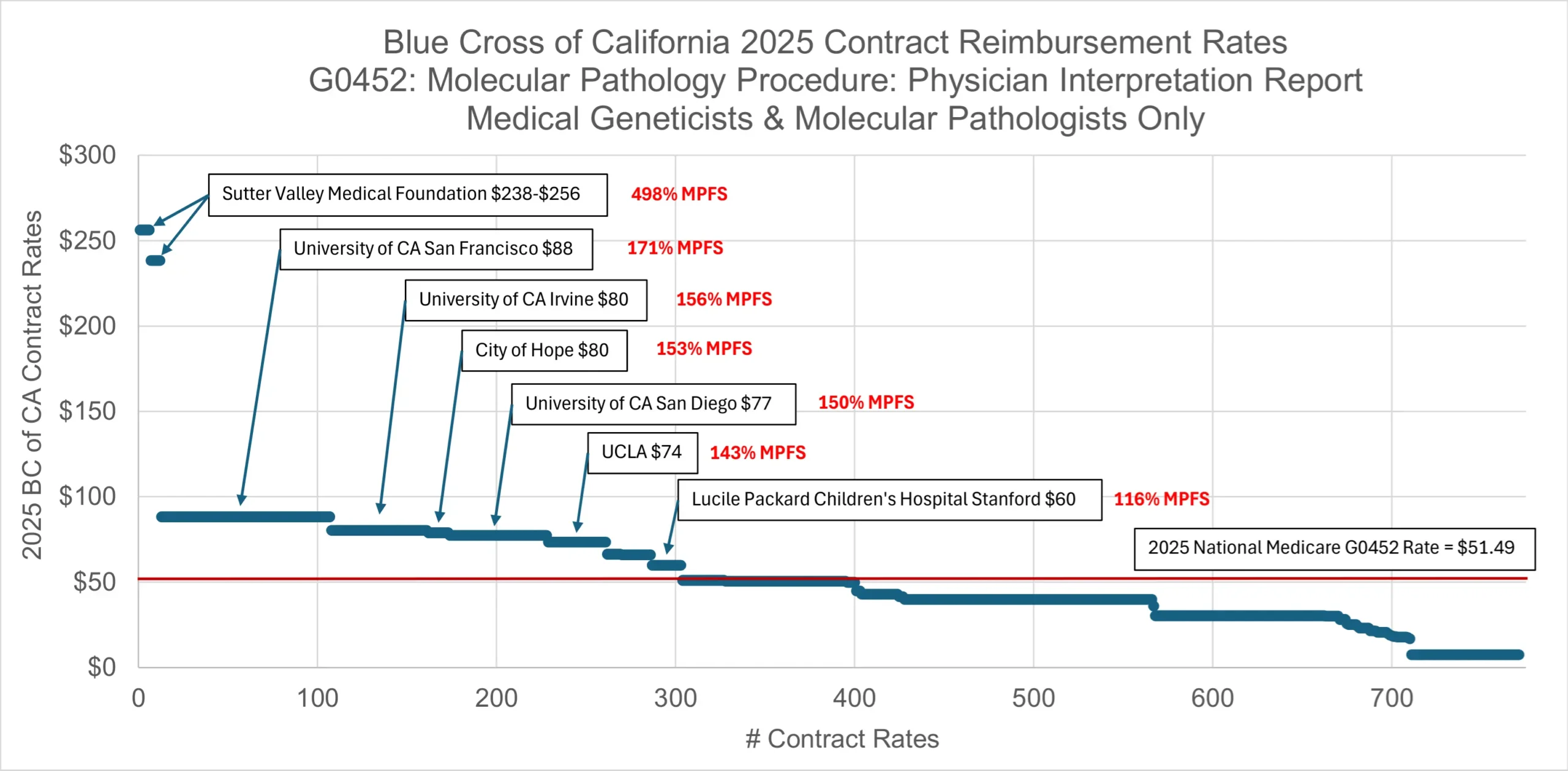
Review of Elevance’s Anthem Blue Cross health plan in California demonstrates similar trends. The median rate for G0452 is about the same as the national Medicare rate for the same service. Large employers of medical geneticists and molecular pathologists have negotiated commercial reimbursement with Anthem Blue Cross of California from 116% (Lucile Packard Children’s Hospital) up to 498% (Sutter Health) of the MPFS.
It is somewhat surprising to see that some major healthcare organizations that employ these specialists, like Rady Children’s Hospital, Cedars Sinai, and Loma Linda School of Medicine all accept reimbursement at or below Medicare for the same service from Anthem Blue Cross of California.
Unless managed care leaders and the market catch up with the growing demand for these specialized services, laboratories that provide genomic and molecular diagnostic tests may need to subsidize the professional expertise necessary to interpret and annotate test results. Documenting Fair Market Value compensation for these services is necessary to comply with the Stark Law exceptions and Anti-Kickback Statute Safe Harbors for these arrangements. Market reimbursement is an important part of the Fair Market Value analysis for these services.